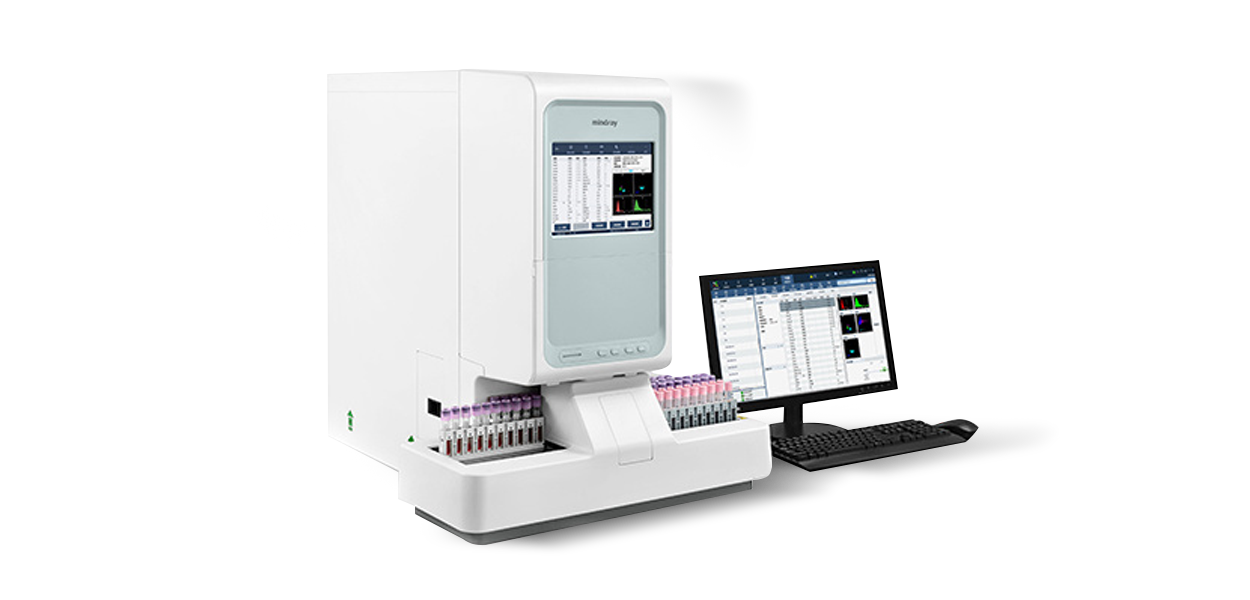
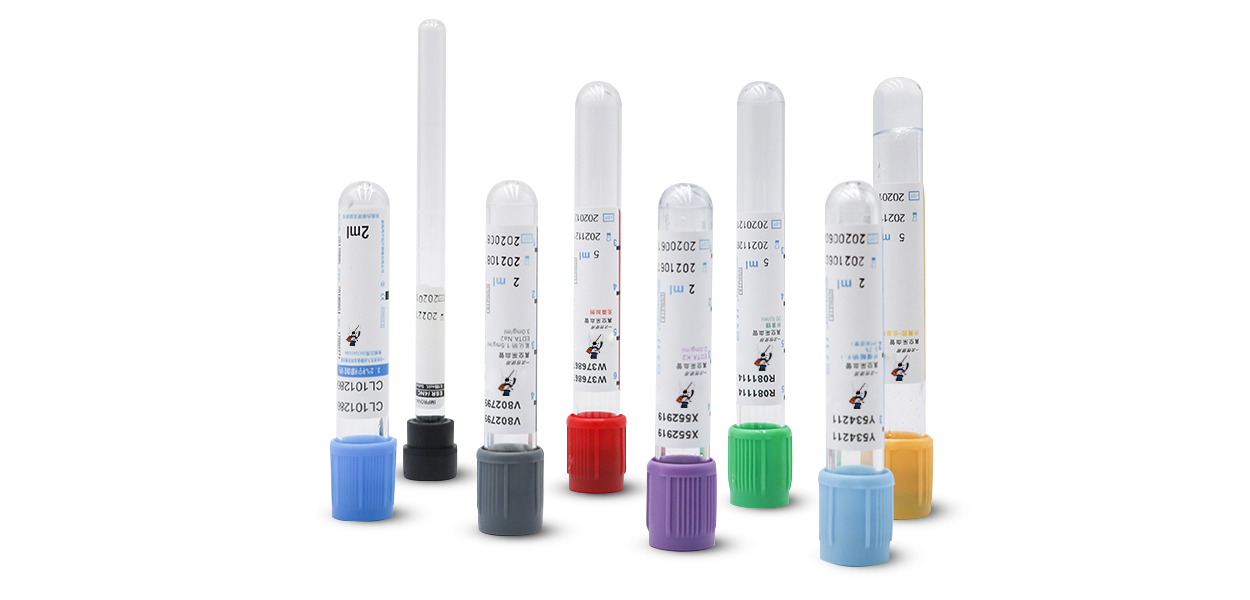
About Us
Company Profile


HuiQuan Medical Equipment Co., LTD
It has a strong R&D technical advantage: the reagents produced can be fully compatible with all blood cell analyzers, biochemical analyzers, immunochemiluminescence analyzers.
Read MoreR&D Experience
Number Of Employees
Area Covered
Partners
Advantage
Deliver Accurate Result to You

Experience
Years of experience in brand industry
Quality is primary concern, HuiQuan team is strict on quality control thus our products have CE, ISO1 3485, ISO9001,CFDA and other international standards certificates.
01

Quality
Whole process control of product quality
Quality is primary concern, HuiQuan team is strict on quality control thus our products have CE, ISO1 3485, ISO9001,CFDA and other international standards certificates.
02

Solution
Full set of technical solutions
Quality is primary concern, HuiQuan team is strict on quality control thus our products have CE, ISO1 3485, ISO9001,CFDA and other international standards certificates.
03

After Sale
Professional after-sales team
Quality is primary concern, HuiQuan team is strict on quality control thus our products have CE, ISO1 3485, ISO9001,CFDA and other international standards certificates.
04
Service Case
Deliver Accurate Result to You
10
2025.12
Unlocking Health Insights: The Versatile Blood Test Kit
Discover the myriad applications of Blood Test Kits, from home health monitoring to professional diagnostics.
03
2025.12
Unlocking Health Insights: The Rise of Blood Test Kits
Discover how blood test kits are revolutionizing personal healthcare and empowering individuals.
26
2025.11
Revolutionizing Healthcare: The Rise of Blood Test Kits
Discover how blood test kits are transforming the healthcare landscape, making diagnosis easier and faster.
19
2025.11
Navigating the World of Blood Test Kits: What You Need to Know
Discover essential tips and considerations for using a Blood Test Kit effectively and safely.
12
2025.11
Demystifying Blood Test Kits: Common Questions Answered
Discover everything you need to know about blood test kits, including how they work, benefits, and FAQs.
10
2025.12
Unlocking Health Insights: The Versatile Blood Test Kit
Discover the myriad applications of Blood Test Kits, from home health monitoring to professional diagnostics.
03
2025.12
Unlocking Health Insights: The Rise of Blood Test Kits
Discover how blood test kits are revolutionizing personal healthcare and empowering individuals.
26
2025.11
Revolutionizing Healthcare: The Rise of Blood Test Kits
Discover how blood test kits are transforming the healthcare landscape, making diagnosis easier and faster.
19
2025.11
Navigating the World of Blood Test Kits: What You Need to Know
Discover essential tips and considerations for using a Blood Test Kit effectively and safely.
12
2025.11
Demystifying Blood Test Kits: Common Questions Answered
Discover everything you need to know about blood test kits, including how they work, benefits, and FAQs.
Service Line
HuiQuan Medical Equipment Co, LTD
Tel.: +86-757-8378-7986
Mobile: +86-15818095185
Email: fshq1680@163.com
WhatsApp:15818095185
广东体达康医疗科技有限公司